ANGSD: Analysis of next generation Sequencing Data
Latest tar.gz version is (0.938/0.939 on github), see Change_log for changes, and download it here.
PCA MDS: Difference between revisions
(→Output) |
|||
| (30 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
This function is new and works from version '''0.912''' and in the latest developmental version from [https://github.com/ANGSD/angsd github] | This function is new and works from version '''0.912''' and in the latest developmental version from [https://github.com/ANGSD/angsd github] | ||
For the PCA / MDS methods you should called SNP sites (use [[PCA]] if you do not want to call SNPs). SNPs can be called based on genotype likelihoods (see [[SNP_calling]]) or you can give the variable sites you want analysis using the [[Sites|-sites]] options. | |||
__TOC__ | __TOC__ | ||
=Brief Overview= | =Brief Overview= | ||
| Line 35: | Line 39: | ||
==Options== | ==Options== | ||
;-doIBS [int] | ;-doIBS [int] | ||
Print a single base from each individual at each position. 1: random sampled read. 2: Consensus base | Print a single base from each individual at each position. 1: random sampled read. 2: Consensus base. If you do not want to print out every site then use -1 or -2. | ||
;doCounts [int] | ;-doCounts [int] | ||
Method requeres counting the different bases at each position. Therefore, -doCounts 1 must be used | Method requeres counting the different bases at each position. Therefore, -doCounts 1 must be used | ||
| Line 46: | Line 50: | ||
Minimum observed minor alleles. The default in 0. If you do not use -doMajorMinor then the number of minor alleles are the sum of the 3 most uncommon alleles. | Minimum observed minor alleles. The default in 0. If you do not use -doMajorMinor then the number of minor alleles are the sum of the 3 most uncommon alleles. | ||
; | ;-minFreq [float] | ||
Minimum minor allele frequency. The default in 0. If you do not use -doMajorMinor then the frequency is the sum of the frequencies of the 3 most uncommon alleles. | Minimum minor allele frequency based on the sampled bases. The default in 0. If you do not use -doMajorMinor then the frequency is the sum of the frequencies of the 3 most uncommon alleles. | ||
;-output01 [int] | ;-output01 [int] | ||
| Line 53: | Line 57: | ||
;-maxMis [int] | ;-maxMis [int] | ||
Maximum missing bases (per site) i.e. maximum number of | Maximum missing bases (per site) i.e. is the maximum number of allowed non-major/minor sampled bases | ||
-makeMatrix [int] | ;-makeMatrix [int] | ||
1 prints out the pairwise IBS matrix. This is the avg. distance between pairs of individuals. Distance is zero if the base in the same and 1 otherwise. | 1 prints out the pairwise IBS matrix. This is the avg. distance between pairs of individuals. Distance is zero if the base in the same and 1 otherwise. You can use this for MDS (see below) | ||
;-doCov [int] | ;-doCov [int] | ||
1 print out the covariance matrix. | 1 print out the covariance matrix which can be used for PCA (see below). You should use the -minFreq option to avoid sites with low allele frequency. | ||
=== run example === | |||
<pre> | |||
./angsd -bam all.files -minMapQ 30 -minQ 20 -GL 2 -doMajorMinor 1 -doMaf 1 -SNP_pval 2e-6 -doIBS 1 -doCounts 1 -doCov 1 -makeMatrix 1 -minMaf 0.05 -P 5 | |||
</pre> | |||
This will produce the output (see below) which includes pairwise differences (.ibsMat) and the covariance matrix (.covMat). These can be used for MDS and PCA respectively (see R example below). Note that only the PCA method require SNP calling and allele frequency estimation. | |||
==Output== | ==Output== | ||
=== sampled bases *ibs.gz === | |||
This function will print the sampled based *ibs.gz. | This function will print the sampled based *ibs.gz. | ||
<div class="toccolours mw-collapsible mw-collapsed"> | <div class="toccolours mw-collapsible mw-collapsed"> | ||
Example of output *.ibs.gz with -doMajorMinor and -output01 1 | Example of output *.ibs.gz with -doMajorMinor>0 and -output01 1 | ||
<pre class="mw-collapsible-content"> | <pre class="mw-collapsible-content"> | ||
chr pos major minor ind0 ind1 ind2 ind3 ind4 ind5 ind6 ind7 | chr pos major minor ind0 ind1 ind2 ind3 ind4 ind5 ind6 ind7 | ||
| Line 123: | Line 138: | ||
'''pos''' is the position | '''pos''' is the position | ||
'''major''' is the major allele | '''major''' is the major allele | ||
'''minor''' is the minor allele | '''minor''' is the minor allele. Needs -doMajorMinor | ||
'''indX''' is the sampled base for individual number X. if -output01 1 then it is 1 for major, 0 for non major and -1 for missing | |||
=== sample based IBS matrix *.ibsMat === | |||
This function will print the pairwise IBS distance | |||
<div class="toccolours mw-collapsible mw-collapsed"> | |||
Example of output *.ibsMat with -makeMatrix 1 | |||
<pre class="mw-collapsible-content"> | |||
0.000000 0.510638 0.606383 0.595745 0.545455 0.428571 | |||
0.510638 0.000000 0.154639 0.154639 0.108911 0.408602 | |||
0.606383 0.154639 0.000000 0.121212 0.137255 0.489362 | |||
0.595745 0.154639 0.121212 0.000000 0.106796 0.484211 | |||
0.545455 0.108911 0.137255 0.106796 0.000000 0.404040 | |||
0.428571 0.408602 0.489362 0.484211 0.404040 0.000000 | |||
0.577320 0.121212 0.181818 0.171717 0.097087 0.473684 | |||
0.536082 0.090000 0.138614 0.118812 0.047619 0.428571 | |||
0.262500 0.571429 0.702381 0.694118 0.632184 0.353659 | |||
0.458333 0.383838 0.484848 0.494949 0.398058 0.368421 | |||
</pre> | |||
</div> | |||
Nind x Nind matrix with pairwise IBS distance | |||
=== sample based covariance matrix *.covMat === | |||
This function will print the covariance matrix based on a single sampled read | |||
<div class="toccolours mw-collapsible mw-collapsed"> | |||
Example of output *.covMat with -doCov 1 | |||
<pre class="mw-collapsible-content"> | |||
1.098251 -0.026225 -0.005617 -0.014726 -0.022438 -0.021786 | |||
-0.026225 1.115986 -0.017167 0.000735 -0.017163 -0.016899 | |||
-0.005617 -0.017167 1.074779 -0.015685 -0.019819 -0.015473 | |||
-0.014726 0.000735 -0.015685 1.072853 -0.013641 -0.007789 | |||
-0.022438 -0.017163 -0.019819 -0.013641 1.094612 -0.016045 | |||
-0.021786 -0.016899 -0.015473 -0.007789 -0.016045 1.059264 | |||
-0.005831 -0.009854 -0.001269 -0.002362 -0.018479 -0.011942 | |||
-0.015399 -0.020010 -0.001296 -0.022947 -0.006515 -0.003938 | |||
-0.001730 -0.040534 -0.002295 -0.017442 -0.024194 -0.007469 | |||
-0.016094 -0.015303 -0.018302 -0.022502 -0.030503 -0.001208 | |||
-0.122045 -0.106068 -0.103089 -0.104443 -0.110237 -0.103610 | |||
-0.106553 -0.100202 -0.104754 -0.109399 -0.107645 -0.111665 | |||
-0.108945 -0.102440 -0.105292 -0.101372 -0.107110 -0.106639 | |||
</pre> | |||
</div> | |||
Nind x Nind covariance matrix | |||
==Model== | ==Model== | ||
=== IBS === | |||
pairwise distance between individuals | |||
<math> | |||
d_{ij} = \frac{\sum_m^M 1-I_{b_j}(b_i)}{M} | |||
</math> | |||
where M in the number of sites with a read for both individuals. <math> 1-I_{b_j}(b_i) </math> is the indicator function which is equal to one with the two individuals i and j have the same base and zero otherwise | |||
=== Covariance === | |||
Allele frequency based on single reads. | |||
<math> | <math> | ||
f_{m} = \frac{N_{minor}}{N_{major} + N_{minor}} | |||
</math> | </math> | ||
<math> | <math> | ||
cov(ij) = \frac{1}{M}\sum_m^M \frac{ (h^i_m-f_m)(h^j_m-f_m) }{f_m(1-f_m)} | |||
</math> | </math> | ||
where M in the number of sites with a read for both individuals. <math> h^i_m</math> is 1 if individuals i for site m has the major allele and zero otherwise | |||
=MDS/PCA using R= | |||
[[File:PCA_MDS.png|thumb]] | |||
<pre> | |||
## MDS | |||
name <- "angsdput.ibsMat" | |||
m <- as.matrix(read.table(name)) | |||
mds <- cmdscale(as.dist(m)) | |||
plot(mds,lwd=2,ylab="Dist",xlab="Dist",main="multidimensional scaling",col=rep(1:3,each=10)) | |||
name <- "angsdput.covMat" | |||
m <- as.matrix(read.table(name)) | |||
e <- eigen(m) | |||
plot(e$vectors[,1:2],lwd=2,ylab="PC 2",xlab="PC 2",main="Principal components",col=rep(1:3,each=10),pch=16) | |||
</pre> | |||
=other fun stuff= | |||
<pre> | |||
## heatmap / clustering / trees | |||
name <- "angsdput.ibsMat" # or covMat | |||
m <- as.matrix(read.table(name)) | |||
#heat map | |||
heatmap(m) | |||
#neighbour joining | |||
plot(ape::nj(m)) | |||
plot(hclust(dist(m), "ave") | |||
</pre> | |||
Latest revision as of 17:26, 20 April 2020
single read sampling approach for PCA or MDS
This function is new and works from version 0.912 and in the latest developmental version from github
For the PCA / MDS methods you should called SNP sites (use PCA if you do not want to call SNPs). SNPs can be called based on genotype likelihoods (see SNP_calling) or you can give the variable sites you want analysis using the -sites options.
Brief Overview
./angsd -doIBS -> angsd version: 0.911-26-gf1cb0e0-dirty (htslib: 1.3-1-gc72ae90) build(Apr 27 2016 11:15:33) -> Analysis helpbox/synopsis information: -> Command: ../angsd/angsd -doIBS -> Wed Apr 27 12:38:35 2016 -------------- abcIBS.cpp: -doIBS 0 (Sampling strategies) 0: no IBS 1: (Sample single base) 2: (Concensus base) -doCounts 0 Must choose -doCount 1 Optional -minMinor 0 Minimum observed minor alleles -minFreq 0.000 Minimum minor allele frequency -output01 0 output 0 and 1s instead of based -maxMis -1 Maximum missing bases (per site) -doMajorMinor 0 use input files or data to select major and minor alleles -makeMatrix 0 print out the ibs matrix -doCov 0 print out the cov matrix
Options
- -doIBS [int]
Print a single base from each individual at each position. 1: random sampled read. 2: Consensus base. If you do not want to print out every site then use -1 or -2.
- -doCounts [int]
Method requeres counting the different bases at each position. Therefore, -doCounts 1 must be used
- -doMajorMinor [int]
The covariance matrix can only be calculated for diallelic sites. Therefore, choose a methods for selecting the major and minor allele (see Inferring_Major_and_Minor_alleles). This can also be use if you only want to make this assumption for the IBS matrix or only want to print out bases that are either the major or minor.
- -minMinor [int]
Minimum observed minor alleles. The default in 0. If you do not use -doMajorMinor then the number of minor alleles are the sum of the 3 most uncommon alleles.
- -minFreq [float]
Minimum minor allele frequency based on the sampled bases. The default in 0. If you do not use -doMajorMinor then the frequency is the sum of the frequencies of the 3 most uncommon alleles.
- -output01 [int]
output the samples reads as 0 (for major) and 1s (for non major) instead of actual base
- -maxMis [int]
Maximum missing bases (per site) i.e. is the maximum number of allowed non-major/minor sampled bases
- -makeMatrix [int]
1 prints out the pairwise IBS matrix. This is the avg. distance between pairs of individuals. Distance is zero if the base in the same and 1 otherwise. You can use this for MDS (see below)
- -doCov [int]
1 print out the covariance matrix which can be used for PCA (see below). You should use the -minFreq option to avoid sites with low allele frequency.
run example
./angsd -bam all.files -minMapQ 30 -minQ 20 -GL 2 -doMajorMinor 1 -doMaf 1 -SNP_pval 2e-6 -doIBS 1 -doCounts 1 -doCov 1 -makeMatrix 1 -minMaf 0.05 -P 5
This will produce the output (see below) which includes pairwise differences (.ibsMat) and the covariance matrix (.covMat). These can be used for MDS and PCA respectively (see R example below). Note that only the PCA method require SNP calling and allele frequency estimation.
Output
sampled bases *ibs.gz
This function will print the sampled based *ibs.gz.
Example of output *.ibs.gz with -doMajorMinor>0 and -output01 1
chr pos major minor ind0 ind1 ind2 ind3 ind4 ind5 ind6 ind7 1 14000873 A G 0 1 1 1 1 1 1 1 14001018 C T 0 1 1 1 1 1 1 1 14001867 G A 0 1 1 1 1 0 1 1 14002342 T C 1 1 1 1 1 -1 1 1 14002422 T A 0 1 1 1 1 0 -1 1 14003581 T C 0 1 1 1 1 1 1 1 14004623 C T 0 1 1 1 1 0 1 1 14006543 T G 0 -1 1 1 1 0 1 1 14007493 G A 0 0 1 -1 1 0 1 1 14007558 T C 0 0 1 1 -1 -1 1 1 14007649 A G 0 1 1 1 1 0 1 1 14008269 A G 1 1 0 -1 1 -1 1
Example of output *.ibs.gz with -doMajorMinor>0 and -output01 0
chr pos major minor ind0 ind1 ind2 ind3 ind4 ind5 ind6 ind7 1 13116 G T N G T T N G N T 1 13118 G A N G A A N G N A 1 14930 A G G G G A N N A N 1 15211 T G N G T G N N N G 1 54490 A G N G N G N N N N 1 54716 T C T C C C T N N N 1 58814 A G N G N G G G N N 1 62777 T A N N A N A A A N 1 63268 C T N T N T C N T N 1 63671 A G N G N N G G G N 1 69428 G T N G T N N T T N 1 69761 T A A A T A N A N N
Example of output *.ibs.gz with -doMajorMinor 0 and -output01 0
chr pos major ind0 ind1 ind2 ind3 ind4 ind5 ind6 ind7 ind8 1 13116 T N G T T N G N T T 1 13118 A N G A A N G N A A 1 14930 A G G G A N N A N G 1 15211 G N G T G N N N G G 1 54490 G N G N G N N N N A 1 54716 C T C C C T N N N C 1 58814 G N G N G G G N N G 1 62777 A N N A N A A A N A 1 63268 T N T N T C N T N N 1 63336 C C C C C C N C N N 1 63671 G N G N N G G G N N
chr is the chromosome
pos is the position
major is the major allele
minor is the minor allele. Needs -doMajorMinor
indX is the sampled base for individual number X. if -output01 1 then it is 1 for major, 0 for non major and -1 for missing
sample based IBS matrix *.ibsMat
This function will print the pairwise IBS distance
Example of output *.ibsMat with -makeMatrix 1
0.000000 0.510638 0.606383 0.595745 0.545455 0.428571 0.510638 0.000000 0.154639 0.154639 0.108911 0.408602 0.606383 0.154639 0.000000 0.121212 0.137255 0.489362 0.595745 0.154639 0.121212 0.000000 0.106796 0.484211 0.545455 0.108911 0.137255 0.106796 0.000000 0.404040 0.428571 0.408602 0.489362 0.484211 0.404040 0.000000 0.577320 0.121212 0.181818 0.171717 0.097087 0.473684 0.536082 0.090000 0.138614 0.118812 0.047619 0.428571 0.262500 0.571429 0.702381 0.694118 0.632184 0.353659 0.458333 0.383838 0.484848 0.494949 0.398058 0.368421
Nind x Nind matrix with pairwise IBS distance
sample based covariance matrix *.covMat
This function will print the covariance matrix based on a single sampled read
Example of output *.covMat with -doCov 1
1.098251 -0.026225 -0.005617 -0.014726 -0.022438 -0.021786 -0.026225 1.115986 -0.017167 0.000735 -0.017163 -0.016899 -0.005617 -0.017167 1.074779 -0.015685 -0.019819 -0.015473 -0.014726 0.000735 -0.015685 1.072853 -0.013641 -0.007789 -0.022438 -0.017163 -0.019819 -0.013641 1.094612 -0.016045 -0.021786 -0.016899 -0.015473 -0.007789 -0.016045 1.059264 -0.005831 -0.009854 -0.001269 -0.002362 -0.018479 -0.011942 -0.015399 -0.020010 -0.001296 -0.022947 -0.006515 -0.003938 -0.001730 -0.040534 -0.002295 -0.017442 -0.024194 -0.007469 -0.016094 -0.015303 -0.018302 -0.022502 -0.030503 -0.001208 -0.122045 -0.106068 -0.103089 -0.104443 -0.110237 -0.103610 -0.106553 -0.100202 -0.104754 -0.109399 -0.107645 -0.111665 -0.108945 -0.102440 -0.105292 -0.101372 -0.107110 -0.106639
Nind x Nind covariance matrix
Model
IBS
pairwise distance between individuals where M in the number of sites with a read for both individuals. is the indicator function which is equal to one with the two individuals i and j have the same base and zero otherwise
Covariance
Allele frequency based on single reads.
where M in the number of sites with a read for both individuals. is 1 if individuals i for site m has the major allele and zero otherwise
MDS/PCA using R
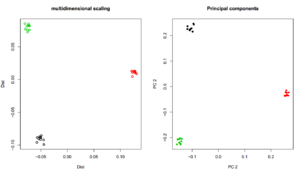
## MDS name <- "angsdput.ibsMat" m <- as.matrix(read.table(name)) mds <- cmdscale(as.dist(m)) plot(mds,lwd=2,ylab="Dist",xlab="Dist",main="multidimensional scaling",col=rep(1:3,each=10)) name <- "angsdput.covMat" m <- as.matrix(read.table(name)) e <- eigen(m) plot(e$vectors[,1:2],lwd=2,ylab="PC 2",xlab="PC 2",main="Principal components",col=rep(1:3,each=10),pch=16)
other fun stuff
## heatmap / clustering / trees name <- "angsdput.ibsMat" # or covMat m <- as.matrix(read.table(name)) #heat map heatmap(m) #neighbour joining plot(ape::nj(m)) plot(hclust(dist(m), "ave")




